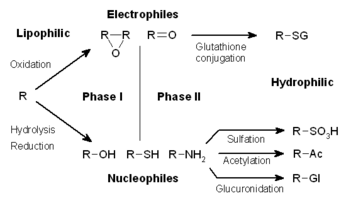
Harmful drug interactions are a major problem all around the world. For example, many people do not realize that eatingeating chocolate, aged cheese, pepperoni, or salami when taking an MAO, monoamine oxidase, inhibitors, like the prescription drugs Nardil or Parnate, could cause a dangerous rise in blood pressure. While doctors and pharmacists are constantly on the lookout for potential problems with the medicines they prescribe, it is still very important for patients to get involved in the discussion....
Read full article Consumers have a responsibility when it comes to using over-the-counter drugs (OTC) and prescription drugs safely. This responsibility can be discharged by following a few simple tips. Always tell your doctor and pharmacist about the drugs you’re currently taking to avoid a drug interaction. Bring a list of drug allergies, dietary supplements, OTC drugs and prescription drugs you take on a regular basis with you to doctors appointments. When you pick up your medications at the pharmacy be sure...
Read full article When you visit a pharmacy, there are some healthy guidelines you should follow to get the most out of your visit. Remember, you may be sitting or standing in a group of other people all waiting for their medications. The pharmacist is multi-tasking by filling prescriptions, speaking with customers about their medicine and overseeing the two or three pharmacy techs. It is important, therefore, to know what to do in order to have as uneventful a visit as possible. Chances are, you’ll stand in...
Read full article Older Americans have the most difficulty of any age group with taking daily medications. Whether they forget a dose, take too much, or don’t understand the food and beverage interactions, the results can be dangerous. Here is a list of factors that contribute to errors when taking medication and some possible solutions. 1. Speaking with a Pharmacist can be frustrating for an older adult with a hearing aid. However, by asking for a written statement of how, and when, to take the medication an...
Read full article Using pain medication in a safe manner is of utmost importance. For, while these drugs are vary effective at controlling pain, pain medication is highly addictive if not taken properly. Always use prescription Pain medication exactly as the label on the prescription bottle indicates. Do not take your medication at the pharmacy unless someone else is driving you immediately home. Some pain drugs work very quickly on an empty stomach.You may feel sleepy, dizzy, lightheaded or drowsy with the medication...
Read full article Most children are not responsible enough to take their medication on their own, so it is up to the caregiver to deliver the medication on time and at the right dose. There are four important reasons why an adult should maintain control on daily prescribed medications. 1. A child will stop taking the medication if they feel better, not realizing that the illness could come back if they do not complete the regimen. 2. Children may take too much if the medication is in liquid form or a sweet chew-able...
Read full article Pharmacists have been answering important questions about the over-the-counter and prescribed medications to quit smoking for decades. Your local pharmacist is available to advise you on Nicotine Replacement Therapy (NRT) and guide you to the treatment that will work best for you. However, until that enlightening conversation with your pharmacist, here are some hints and tips to assist you in your attempt to quit smoking. First, make sure to get your medications in order before you actually stop...
Read full article If you are old enough to qualify for Medicare and its prescription program, then you are probably old enough to appreciate some hints about how to use this new privilege. This is your time to enjoy the world on your own terms, and the last thing you want is to be delayed at the pharmacy. Your Medicare card will show, among other things, which of the Medicare programs you chose. Therefore, when you visit the pharmacy, make sure you have your Medicare card with you. You will also need the Medicare...
Read full article Pharmaceutical companies often change the color, shape, and dosage of medications. It is the pharmacist’s job is to keep up with these changes so he or she can dispense your medication quickly and accurately. Help you pharmacist by being proactive about your health and following these easy steps: 1. Always ask your doctor why you are being prescribed a given medication. Know the purpose, food and beverage interactions, and potential problems with any of the other drugs you may be taking. 2....
Read full article Just because you need a prescription medication, doesn’t mean you can’t shop around to get the best price. Shopping around is particularly valuable if you are on a given medication for an extended period of time. However, these tips and tricks can be used to save money any time you get a prescription: 1. Always check for prescription transfer coupons. Some pharmacies offer as much as $20-$30 in bonus cards if you transfer all your drugs to their pharmacy. However, make sure all your drugs...
Read full article